Shop
Top Selling Products
RETATRUTIDE 10MG (1 VIAL): The Triple Agonist That’s Outperforming Tirzepatide

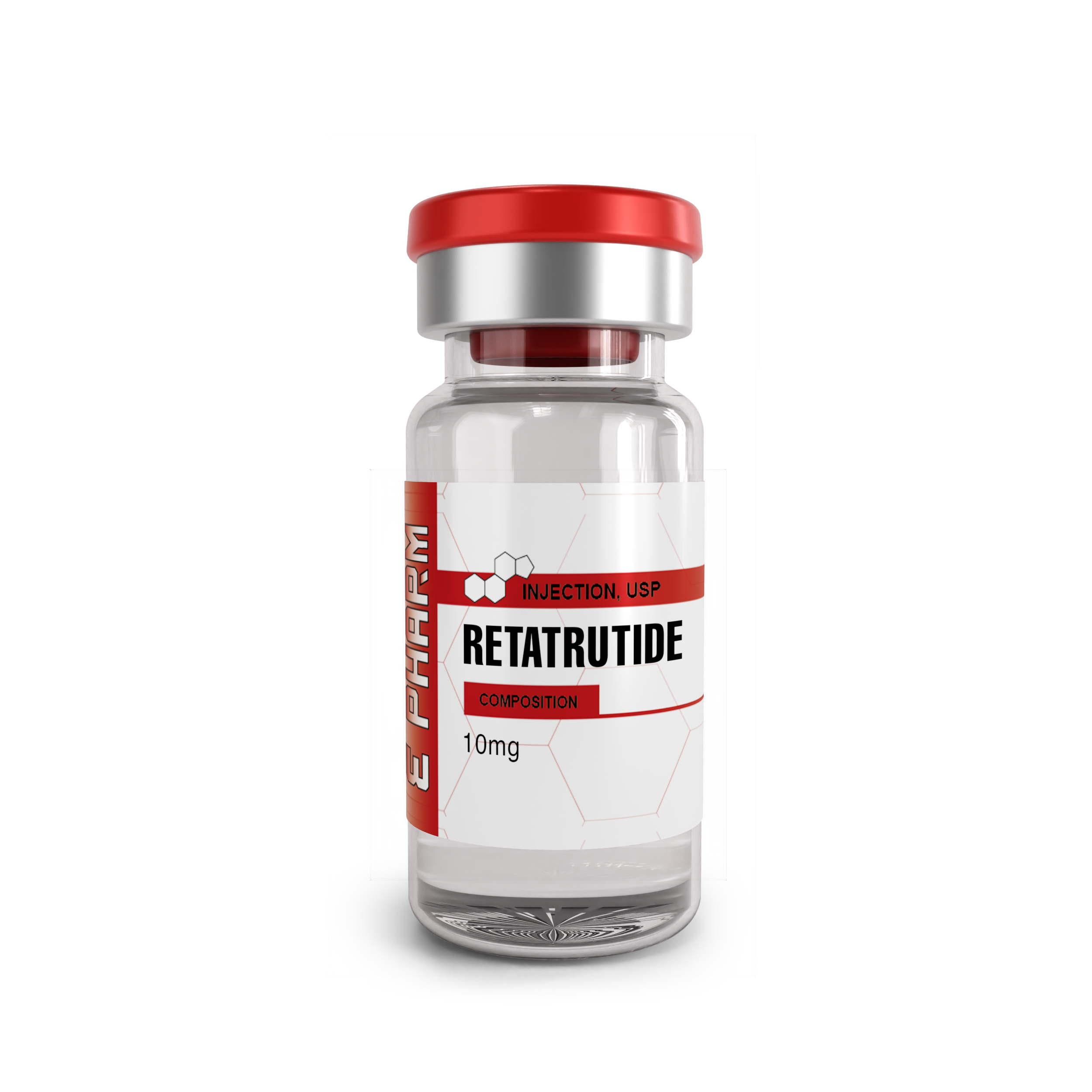
RETATRUTIDE 10MG (1 VIAL): The Triple Agonist That’s Outperforming Tirzepatide
$99.99
Reta is an investigational drug, still in clinical trials, that acts as a triple agonist targeting three different receptors: glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon receptors.
- Free Shipping & Exchanges
- Flexible and secure payment, pay on delivery
- 600,000 happy customers
Description
Reta 10MG (1 Vial) – Buy Online in the USA | Limited-Time Offer
Reta 10MG from ASN-LABS is a high-quality research peptide available in a sterile 1-vial format. Known for its potential in metabolic and weight management studies, Reta is lab-tested to ensure purity and reliability. Perfect for researchers seeking consistent results, this product comes with fast, discreet shipping across the USA, including all 50 states.
Key Features of Reta 10MG:
-
Potency: 10MG (1 vial)
-
Use: Research peptide for metabolic studies
-
Purity: Third-party lab-tested
-
Nationwide USA Shipping – Discreet & secure
Reta is an investigational drug, still in clinical trials, that acts as a triple agonist targeting three different receptors: glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon receptors.
Here’s a closer look at reta:
- Mechanism of action: It works by mimicking the actions of the natural gut hormones GLP-1, GIP, and glucagon, which play crucial roles in regulating blood sugar, appetite, and metabolism. This triple agonism contributes to increased insulin secretion, improved glucose homeostasis, appetite modulation, and increased energy expenditure.
- Potential uses: It is being investigated for the treatment of obesity and type 2 diabetes.
- Side effects: Common side effects are mainly gastrointestinal and include nausea, vomiting, diarrhea, and constipation, says canadianinsulin.com. These are typically mild to moderate and can be managed with dose adjustments.
- Availability: Reta is not yet FDA approved. It is currently in Phase 3 clinical trials, which are expected to conclude in early 2026. If approved, it could be available by 2027.
- Administration: It’s administered as a weekly subcutaneous injection, typically starting with a lower dose that’s gradually increased.
Disclaimer: For Research Purposes Only
This content is provided strictly for research purposes and does not constitute an endorsement or recommendation for the non-laboratory application or improper handling of peptides designed for research. The information, including discussions about specific peptides and their researched benefits, is presented for informational purposes only and must not be construed as health, clinical, or legal guidance, nor an encouragement for non-research use in humans. Peptides described here are solely for use in structured scientific study by authorized individuals. We advise consulting with research experts, medical practitioners, or legal counsel prior to any decisions about obtaining or utilizing these peptides. The expectation of responsible, ethical utilization of this information for legitimate investigative and scholarly objectives is paramount. This notice is dynamic and governs all provided content on research peptides.
Additional information
| Weight | .001 oz |
|---|---|
| Dimensions | 5 × 5 × 5 cm |
Reviews (0)
Be the first to review “RETATRUTIDE 10MG (1 VIAL): The Triple Agonist That’s Outperforming Tirzepatide” Cancel reply
Shipping & Delivery































Reviews
There are no reviews yet.